 |
ESTROGENS INCREASE THE RISK OF
|
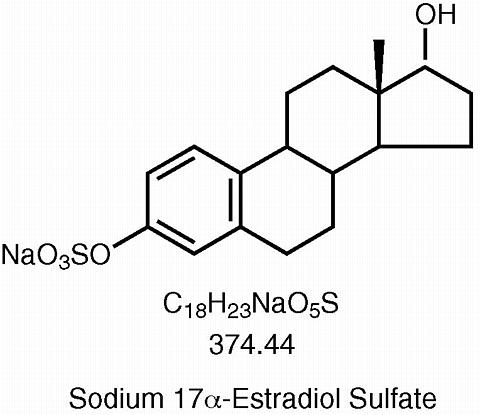
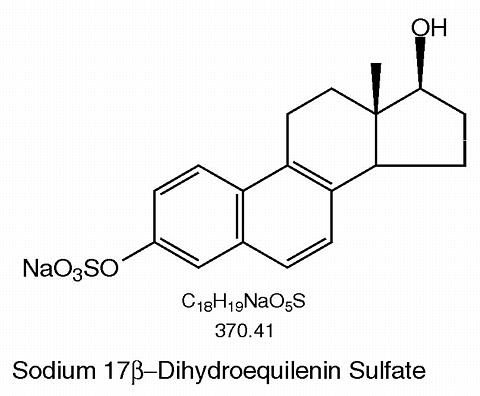
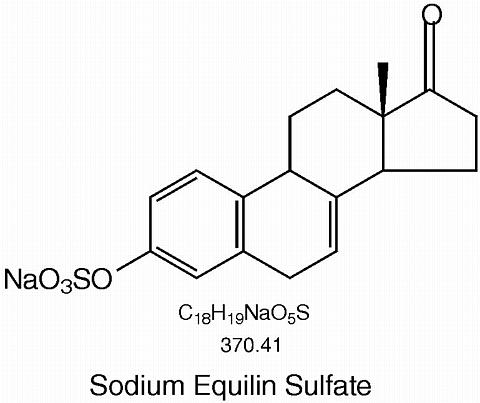
Synthetic conjugated estrogens, A tablets contain a blend of nine (9) synthetic estrogenic substances. The estrogenic substances are sodium estrone sulfate, sodium equilin sulfate, sodium 17(alpha)-dihydroequilin sulfate, sodium 17(alpha)-estradiol sulfate, sodium 17(beta)-dihydroequilin sulfate, sodium 17(alpha)-dihydroequilenin sulfate, sodium 17(beta)-dihydroequilenin sulfate, sodium equilenin sulfate and sodium 17(beta)-estradiol sulfate.
The structural formulae for these estrogens are:
 |
 |
 |
 |
 |
 |
 |
 |
 |
Tablets for oral administration, are available in 0.625 mg, 0.9 mg and 1.25 mg strengths of synthetic conjugated estrogens. Tablets also contain the following inactive ingredients: ethylcellulose, hydroxypropyl methylcellulose, lactose monohydrate, magnesium stearate, polyethylene glycol, polysorbate 80, pregelatinized starch, titanium dioxide, and triethyl citrate.
-0.625 mg tablets also contain: FD&C Red No. 40 aluminum lake.
-0.9 mg tablets do not contain any color additives.
-1.25 mg tablets also contain: FD&C Blue No. 2 aluminum lake.
Estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrogen and is substantially more potent than its metabolites, estrone and estriol at the receptor level. The primary source of estrogen in normally cycling adult women is the ovarian follicle, which secretes 70 to 500 µg of estradiol daily, depending on the phase of the menstrual cycle. After menopause, most endogenous estrogen is produced by conversion of androstenedione, secreted by the adrenal cortex, to estrone by peripheral tissues. Thus, estrone and the sulfate conjugated form, estrone sulfate, are the most abundant circulating estrogens in postmenopausal women.
Circulating estrogens modulate the pituitary secretion of the gonadotropins, leutinizing hormone (LH) and follicle stimulating hormone (FSH) through a negative feedback mechanism and estrogen replacement therapy acts to reduce the elevated levels of these hormones seen in postmenopausal women.
Synthetic conjugated estrogens are soluble in water and are well absorbed from the gastrointestinal tract after release from the drug formulation. The Cenestin tablet releases the synthetic conjugated estrogens, A slowly over a period of several hours. Maximum plasma concentrations of conjugated estrogens are attained at about 8 hours and unconjugated estrogens are attained at about 9 hours after oral administration.
|
||||||||||||||||||||||||||||||||||||||||
 |
The effect of food on the 0.625, 0.9 and 1.25 mg tablets has not been studied.
The distribution of exogenous estrogens is similar to that of endogenous estrogens. Estrogens are widely distributed in the body and are generally found in higher concentrations in the sex hormone target organs. Estradiol and other naturally occurring estrogens are bound mainly to sex hormone binding globulin (SHBG), and to a lesser degree to albumin. Conjugated estrogens bind mainly to albumin while the unconjugated estrogens bind to both albumin and sex-hormone binding globulin (SHBG).
Exogenous estrogens are metabolized in the same manner as endogenous estrogens. Circulating estrogens exist in a dynamic equilibrium of metabolic interconversions. These transformations take place mainly in the liver. Estradiol is converted reversibly to estrone, and both can be converted to estriol, which is the major urinary metabolite. Estrogens also undergo enterohepatic recirculation via sulfate and glucuronide conjugation in the liver, biliary secretion of conjugates into the intestine, and hydrolysis in the gut followed by reabsorption. In postmenopausal women a significant portion of the circulating estrogens exist as sulfate conjugates, especially estrone sulfate, which serves as a circulating reservoir for the formation of more active estrogens.
Estradiol, estrone, and estriol are excreted in the urine along with glucuronide and sulfate conjugates. The mean (SD) apparent terminal elimination half-life (t ½ ) of conjugated estrone is 15 (± 9) hours and conjugated equilin is 10 (± 3) hours.
There are no known drug interactions with estrogens.
A randomized, placebo-controlled multicenter clinical study was conducted evaluating the effectiveness of Cenestin for the treatment of vasomotor symptoms in 120 menopausal women. Patients were randomized to receive either placebo or 0.625 mg Cenestin daily for 12 weeks. Dose titration was allowed after one week of treatment. The starting dose was either doubled (2 × 0.625 mg Cenestin or placebo taken daily) or reduced (0.3 mg Cenestin or placebo taken daily), if necessary. Efficacy was assessed at 4, 8 and 12 weeks of treatment. By Week 12, 10% of the study participants remained on a single 0.625 mg Cenestin tablet daily while 77% required two (0.625 mg) tablets daily. The results in Table 2 indicate that compared to placebo, Cenestin produced a reduction in moderate-to-severe vasomotor symptoms at all time points (4, 8, and 12 weeks).
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Cenestin (synthetic conjugated estrogens, A) Tablets are indicated in the treatment of moderate to severe vasomotor symptoms associated with the menopause.
Estrogens should not be used in individuals with any of the following conditions:
1. Addition of a progestin when a woman has not had a hysterectomy
Studies of the addition of a progestin for 10 or more days of a cycle of estrogen administration, or daily with estrogen in a continuous regimen, have reported a lowered incidence of endometrial hyperplasia than would be induced by estrogen treatment alone.
There are, however, possible risks which may be associated with the use of progestins in estrogen replacement regimens. These include:
(a)adverse effects on lipoprotein metabolism (lowering HDL and raising LDL)
(b)impairment of glucose tolerance; and
(c)possible enhancement of mitotic activity in breast epithelial tissue, although few epidemiological data are available to address this point.
The choice of progestin, its dose, and its regimen may be important in minimizing these adverse effects.
Substantial increases in blood pressure during estrogen replacement therapy have been attributed to idiosyncratic reactions to estrogens in a small number of case reports. A generalized effect of estrogen therapy on blood pressure was not found in the one randomized, placebo-controlled study that has been reported.
3. Familial hyperlipoproteinemia
Estrogen therapy may be associated with elevations of plasma triglycerides leading to pancreatitis and other complications in patients with familial defects of lipoprotein metabolism.
Estrogens may be poorly metabolized in patients with impaired liver function.
See text of PATIENT LABELING, below.
Estrogen administration should generally be guided by clinical response at the smallest dose, rather than laboratory monitoring, for relief of symptoms for those indications in which symptoms are observable.
1. Accelerated prothrombin time, partial thromboplastin time, and platelet aggregation time; increased platelet count; increased factors II, VII antigen, VIII antigen, VIII coagulant activity, IX, X, XII, VII-X complex, II-VII-X complex, and beta-thromboglobulin; decreased levels of anti-factor Xa and antithrombin III, decreased antithrombin III activity; increased levels of fibrinogen and fibrinogen activity; increased plasminogen antigen and activity.
2. Increased thyroid-binding globulin (TBG) leading to increased circulating total thyroid hormone, as measured by protein-bound iodine (PBI), T4 levels (by column or by radioimmunoassay) or T3 levels by radioimmunoassay. T3 resin uptake is decreased, reflecting the elevated TBG. Free T4 and free T3 concentrations are unaltered.
3. Other binding proteins may be elevated in serum, i.e., corticosteroid binding globulin (CGB), sex hormone-binding globulin (SHBG), leading to increased circulating corticosteroids and sex steroids respectively. Free or biologically active hormone concentrations are unchanged. Other plasma proteins may be increased (angiotensinogen/renin substrate, alpha-1-antitrypsin, ceruloplasmin).
4. Increased plasma HDL and HDL-2 subfraction concentrations, reduced LDL cholesterol concentration, increased triglycerides levels.
5. Impaired glucose tolerance.
6. Reduced response to metyrapone test.
7. Reduced serum folate concentration.
Long-term continuous administration of natural and synthetic estrogens in certain animal species increases the frequency of carcinomas of the breast, uterus, cervix, vagina, testis, and liver. See CONTRAINDICATIONS and WARNINGS .
Estrogens are not indicated for use during pregnancy or the immediate postpartum period. Estrogens are ineffective for the prevention or treatment of threatened or habitual abortion. Treatment with diethylstibestrol (DES) during pregnancy has been associated with an increased risk of congenital defects and cancer in the reproductive organs of the fetus, and possibly other birth defects. The use of DES during pregnancy has also been associated with a subsequent increased risk of breast cancer in the mothers.
As a general principle, the administration of any drug to nursing mothers should be done only when clearly necessary since many drugs are excreted in human milk. In addition, estrogen administration to nursing mothers has been shown to decrease the quantity and quality of the milk. Estrogens are not indicated for the prevention of postpartum breast engorgement.
Safety and efficacy of Cenestin for the treatment of vasomotor symptoms due to hypoestrogenism in pediatric patients have not been established.
See WARNINGS and PRECAUTIONS regarding the potential adverse effects on the fetus, the induction of malignant neoplasms, gallbladder disease, cardiovascular disease, elevated blood pressure and hypercalcemia. In a 12-week clinical trial that included 72 women treated with Cenestin and 48 women treated with placebo, the following adverse events occurred at a rate >/= 5% (see Table 3).
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The following additional adverse reactions have been reported with estrogen therapy:
Serious ill effects have not been reported following acute ingestion of large doses of estrogen-containing products by young children. Overdosage of estrogen may cause nausea and vomiting, and withdrawal bleeding may occur in females.
For treatment of moderate to severe vasomotor symptoms associated with the menopause, the lowest dose and regimen that will control symptoms should be chosen. Initial doses of 0.625 mg are recommended with titration up to 1.25 mg. Medication should be discontinued as promptly as possible. Attempts to discontinue or taper medication should be made at 3-month to 6-month intervals.
Cenestin (synthetic conjugated estrogens, A) Tablets,
-0.625 mg tablets are available in containers of 30 (NDC 51285-442-30), 100 (NDC 51285-442-02), and 1000 (NDC 51285-442-05).
Tablets are round, red colored, film-coated, and are debossed with letters, dp , and number, 42.
-0.9 mg tablets are available in containers of 30 (NDC 51285-443-30), 100 (NDC 51285-443-02), and 1000 (NDC 51285-443-05).
Tablets are round, white, film-coated, and are debossed with letters, dp , and number, 43.
-1.25 mg tablets are available in containers of 30 (NDC 51285-444-30), 100 (NDC 51285-444-02), and 1000 (NDC 51285-444-05).
Tablets are round, blue colored, film-coated, and are debossed with letters, dp , and number, 44.
Store at 25°C (77°F); excursions are permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature]
Dispense in tight container as defined in USP.
Dispense in child-resistant packaging.
Dispenser: Include one "Information for the patient" leaflet with each package dispensed.
Cenestin®
(synthetic conjugated estrogens, A) Tablets
This leaflet describes when and how to use estrogens, and the risks and benefits of estrogen treatment.
Estrogens have important benefits but also some risks. You must decide, with your doctor, whether the risks are acceptable in comparison to the benefits. If you use estrogens, check with your doctor to be sure you are using the lowest possible dose that works, and that you don't use them longer than necessary. How long you need to use estrogens will depend on the reasons for use.
ESTROGENS INCREASE THE RISK OF CANCER OF THE UTERUSIf you use any drug that contains estrogen, it is important to visit your doctor regularly and report any unusual vaginal bleeding right away. Vaginal bleeding after menopause may be a warning sign of uterine cancer. Your doctor should evaluate any unusual vaginal bleeding to find out the cause. |
To reduce moderate or severe menopausal symptoms.
Estrogens are hormones made by the ovaries of normal women. Between ages 45 and 55, the ovaries normally stop making estrogens. This leads to a drop in body estrogen levels which causes the "change of life" or menopause (the end of monthly menstrual periods). If both ovaries are removed during an operation before natural menopause takes place, the sudden drop in estrogen levels causes "surgical menopause".
When estrogen levels begin dropping, some women develop very uncomfortable symptoms, such as feelings of warmth in the face, neck and chest or sudden intense episodes of heat and sweating ("hot flashes" or "hot flushes"). Using estrogen drugs can help the body adjust to lower estrogen levels and reduce these symptoms. Most women have only mild menopausal symptoms or none at all and do not need to use estrogen drugs for these symptoms. Others may need to take estrogens for a few months while their bodies adjust to lower estrogen levels. The majority of women do not need estrogen replacement for longer than six months for these symptoms.
Cenestin should not be used:
In addition to the risks listed above, the following side effects have been reported with estrogen use:
Cenestin has not been shown either effective or safe for use by infants, children, or adolescent boys or girls.
If you use estrogens, you can reduce your risks by doing these things:
Estrogens increase the risk of developing a condition (endometrial hyperplasia) that may lead to cancer of the lining of the uterus. Taking progestins, another hormone drug, with estrogens lowers the risk of developing this condition. Therefore, if your uterus has not been removed, your doctor may prescribe a progestin for you to take together with the estrogen.
Your doctor has prescribed this drug for you and you alone. Do not give the drug to anyone else.
Keep this and all drugs out of the reach of children. In case of overdose, call your doctor, hospital or poison control center immediately.
Cenestin (synthetic conjugated estrogens, A) Tablets,
-0.625 mg tablets are available in containers of 30 (NDC 51285-442-30), 100 (NDC 51285-442-02), and 1000 (NDC 51285-442-05).
Tablets are round, red colored, film-coated, and are debossed with letters, dp , and number, 42.
-0.9 mg tablets are available in containers of 30 (NDC 51285-443-30), 100 (NDC 51285-443-02), and 1000 (NDC 51285-443-05).
Tablets are round, white, film-coated, and are debossed with letters, dp , and number, 43.
-1.25 mg tablets are available in containers of 30 (NDC 51285-444-30), 100 (NDC 51285-444-02), and 1000 (NDC 51285-444-05).
Tablets are round, blue colored, film-coated, and are debossed with letters, dp , and number, 44.
Store at 25°C (77°F); excursions are permitted to 15°-30°C (59°-86°F). [See USP Controlled Room Temperature]
Manufactured by:Duramed Pharmaceuticals, Inc.
Cincinnati, OH 45213 USA
I00379B REV. 01/00